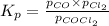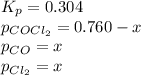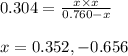Chemistry, 02.08.2019 23:20 meadowsoares7
The equilibrium constant kc for the decomposition of phosgene, cocl2, is 4.63x10^-3 at 527 c. calculate the equilibrium partial pressure of all the components, starting with pure phosgene at 0.760 atm

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
The equilibrium constant kc for the decomposition of phosgene, cocl2, is 4.63x10^-3 at 527 c. calcul...
Questions

Biology, 19.09.2019 03:00



Mathematics, 19.09.2019 03:00

Mathematics, 19.09.2019 03:00

Mathematics, 19.09.2019 03:00


Mathematics, 19.09.2019 03:00


History, 19.09.2019 03:00




Social Studies, 19.09.2019 03:00

Mathematics, 19.09.2019 03:00


Mathematics, 19.09.2019 03:00

Mathematics, 19.09.2019 03:00

English, 19.09.2019 03:00

English, 19.09.2019 03:00

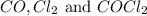 are 0.352 atm, 0.352 atm and 0.408 atm respectively.
are 0.352 atm, 0.352 atm and 0.408 atm respectively.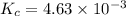
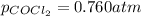
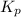 with
with 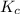 is given by the formula:
is given by the formula: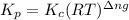
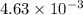
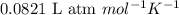
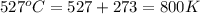
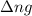 = change in number of moles of gas particles =
= change in number of moles of gas particles = 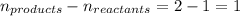
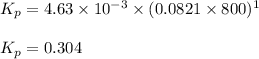
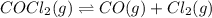
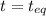 0.760-x x x
0.760-x x x