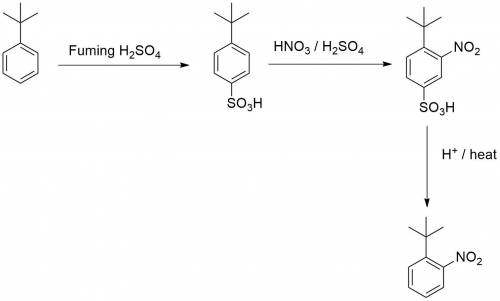
Chemistry, 02.08.2019 23:20 kkeith121p6ujlt
Knowing that the sulfonation of benzenes with sulfuric acid is a reversible process, explain how you could leverage this to allow you to make just 1-(1,1-dimethylethyl)-2-nitrobenzen e from (1,1-dimethylethyl)benzene.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
Knowing that the sulfonation of benzenes with sulfuric acid is a reversible process, explain how you...
Questions

Mathematics, 12.01.2021 17:30

Mathematics, 12.01.2021 17:30

Mathematics, 12.01.2021 17:30





Biology, 12.01.2021 17:30


Mathematics, 12.01.2021 17:30



Mathematics, 12.01.2021 17:30

History, 12.01.2021 17:30


Mathematics, 12.01.2021 17:30



Chemistry, 12.01.2021 17:30

Mathematics, 12.01.2021 17:30

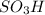 groups add onto para position to 1,1-dimethylethyl group due to electron donating effect and bulky size of 1,1-dimethylethyl group.
groups add onto para position to 1,1-dimethylethyl group due to electron donating effect and bulky size of 1,1-dimethylethyl group.