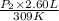Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
Consider 4.60 l of a gas at 365 mmhg and 20 c . if the container is compressed to 2.60 l and the tem...
Questions

Health, 08.05.2021 03:50

Chemistry, 08.05.2021 03:50


Computers and Technology, 08.05.2021 03:50




Mathematics, 08.05.2021 03:50



Social Studies, 08.05.2021 03:50



Computers and Technology, 08.05.2021 03:50



Biology, 08.05.2021 03:50

Spanish, 08.05.2021 03:50

Mathematics, 08.05.2021 03:50

Mathematics, 08.05.2021 03:50

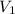 = 4.60 L,
= 4.60 L, 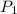 = 365 mm Hg
= 365 mm Hg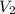 = 2.60 L,
= 2.60 L, 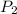 = ?
= ?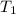 = (20 + 273) K = 293 K,
= (20 + 273) K = 293 K, 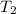 = (36 + 273) K = 309 K
= (36 + 273) K = 309 K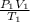 =
= 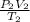
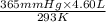 =
=