
Chemistry, 03.08.2019 00:10 babydoll1981
Carbon disulfide is a colorless liquid. when pure, it is nearly odorless, but the commercial product smells vile. carbon disulfide is used in the manufacture of rayon and cellophane. the liquid burns as follows: cs2(l) + 3o2(g) → co2(g) + 2so2(g)calculate the standard enthalpy change for this reaction usingstandard enthalpies of formation.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1
You know the right answer?
Carbon disulfide is a colorless liquid. when pure, it is nearly odorless, but the commercial product...
Questions

Mathematics, 22.10.2020 20:01




History, 22.10.2020 20:01


Chemistry, 22.10.2020 20:01

History, 22.10.2020 20:01


Mathematics, 22.10.2020 20:01


Mathematics, 22.10.2020 20:01



English, 22.10.2020 20:01


Geography, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01

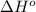
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0163/6329/45485.png)
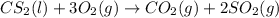
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(CO_2)})+(2\times \Delta H^o_f_{(SO_2)})]-[(1\times \Delta H^o_f_{(CS_2)})+(3\times \Delta H^o_f_{(O_2)})]](/tpl/images/0163/6329/025c0.png)
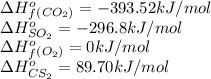
![\Delta H^o_{rxn}=[(1\times (-393.52))+(2\times (-296.8))]-[(1\times (89.70))+(3\times (0)]\\\\\Delta H^o_{rxn}=-1076.82kJ](/tpl/images/0163/6329/16879.png)



