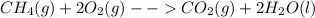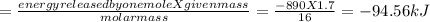Chemistry, 03.08.2019 00:20 jtorres0520
In the presence of excess oxygen, methane gas burns in a constant-pressure system to yield carbon dioxide and water: ch4 (g) 2o2 (g) → co2 (g) 2h2o(l) δh = -890.0 kj
calculate the value of q (kj) in this exothermic reaction when 1.70 g of methane is combusted at constant pressure.
(a) -94.6 kj
(b) 0.0306 kj
(c) -0.0106 kj
(d) 32.7 kj
(e) -9.46 × 10^4 kj

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
In the presence of excess oxygen, methane gas burns in a constant-pressure system to yield carbon di...
Questions



Mathematics, 09.02.2022 05:10



History, 09.02.2022 05:10


SAT, 09.02.2022 05:10

Geography, 09.02.2022 05:10


Physics, 09.02.2022 05:10



Computers and Technology, 09.02.2022 05:10


Mathematics, 09.02.2022 05:10


Mathematics, 09.02.2022 05:10


SAT, 09.02.2022 05:10