Chemistry, 03.08.2019 00:30 trinigal83
The useful metal manganese can be extracted from the mineral rhodochrosite by a two-step in the first step, manganese(ii) carbonate and oxygen react to form manganese(iv) oxide and carbon dioxide: 2mnco3 + o2=2mno2 + 2co2in the second step, manganese(iv) oxide and aluminum react to form manganese and aluminum oxidide: 3mno2 + 4al = 3mn + 2al2o3 suppose the yield of the first step is 65.% and the yield of the second step is 80.%. calculate the mass of manganese(ii) carbonate required to make 8.0kg of manganese. be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2


Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1
You know the right answer?
The useful metal manganese can be extracted from the mineral rhodochrosite by a two-step in the fir...
Questions

Mathematics, 23.03.2021 03:30


Chemistry, 23.03.2021 03:30


Mathematics, 23.03.2021 03:30


Mathematics, 23.03.2021 03:30


English, 23.03.2021 03:30


Mathematics, 23.03.2021 03:30


Mathematics, 23.03.2021 03:30

Mathematics, 23.03.2021 03:30




English, 23.03.2021 03:30

Mathematics, 23.03.2021 03:30

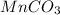 required are, 35 kg
required are, 35 kg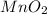 .
.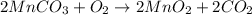
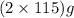 of
of 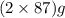 of
of 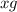 of
of 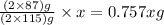 of
of 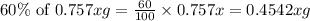
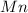 .
.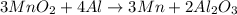
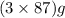 of
of 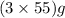 of
of 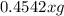 of
of 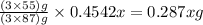 of
of