
Chemistry, 03.08.2019 01:10 BIGJAYWESTSIDE
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g n2o4 and 45.0 g n2h4. some possibly useful molar masses are as follows: n2o4 = 92.02 g/mol, n2h4 = 32.05 g/mol.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2
You know the right answer?
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g...
Questions

Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01



Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01

Chemistry, 20.09.2020 04:01




English, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01

Social Studies, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01


Chemistry, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01

Business, 20.09.2020 04:01

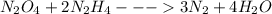
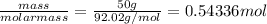
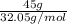 =1.4041mol
=1.4041mol


