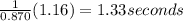Chemistry, 03.08.2019 03:20 ariestburks0513
The rate constant for this first‑order reaction is0.870 s−1 at 400 ∘c. a⟶products how long, in seconds, would it take for the concentration of a to decrease from 0.830 m to 0.260 m?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
The rate constant for this first‑order reaction is0.870 s−1 at 400 ∘c. a⟶products how long, in secon...
Questions


Mathematics, 03.04.2020 00:50



Advanced Placement (AP), 03.04.2020 00:51




Mathematics, 03.04.2020 00:51

Mathematics, 03.04.2020 00:51




Advanced Placement (AP), 03.04.2020 00:51


Mathematics, 03.04.2020 00:51



Mathematics, 03.04.2020 00:51

Mathematics, 03.04.2020 00:51

![kt = ln \frac{[A_{0}]}{[A_{t}]}](/tpl/images/0164/1271/da030.png)
](/tpl/images/0164/1271/35424.png)