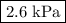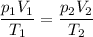Chemistry, 04.08.2019 05:10 kprincess16r
Asample of nitrogen is initially at a pressure of 1.7 kpa, a temperature of -10 c and a volume of 7.5 m3. then the volume is decreased to 3.8 m3. the temperature is decreased to 200 k. what is the final pressure of the nitrogen gas?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1
You know the right answer?
Asample of nitrogen is initially at a pressure of 1.7 kpa, a temperature of -10 c and a volume of 7....
Questions

Mathematics, 03.05.2020 13:51




Computers and Technology, 03.05.2020 13:51

Mathematics, 03.05.2020 13:51

Mathematics, 03.05.2020 13:51

Mathematics, 03.05.2020 13:51


Mathematics, 03.05.2020 13:51



Mathematics, 03.05.2020 13:51


Biology, 03.05.2020 13:51



Mathematics, 03.05.2020 13:51

Mathematics, 03.05.2020 13:51

Geography, 03.05.2020 13:51