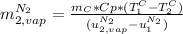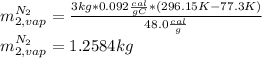Chemistry, 05.08.2019 17:10 michaelwthms
A3.00-kg block of copper at 23.0°c is dropped into a large vessel of liquid nitrogen at 77.3 k. how many kilograms of nitrogen boil away by the time the copper reaches 77.3 k? (the specific heat of copper is 0.092 0 cal/g · °c, and the latent heat of vaporization of nitrogen is 48.0 cal/g.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 06:30
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2
You know the right answer?
A3.00-kg block of copper at 23.0°c is dropped into a large vessel of liquid nitrogen at 77.3 k. how...
Questions

Mathematics, 16.07.2019 22:00

English, 16.07.2019 22:00

History, 16.07.2019 22:00

Social Studies, 16.07.2019 22:00



Social Studies, 16.07.2019 22:00




Mathematics, 16.07.2019 22:00




Social Studies, 16.07.2019 22:00

Biology, 16.07.2019 22:00

History, 16.07.2019 22:00


Mathematics, 16.07.2019 22:00

Biology, 16.07.2019 22:00

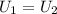
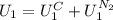
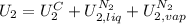
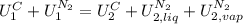
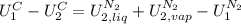
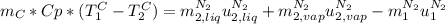
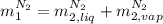 ,
,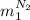 in the energy balance:
in the energy balance: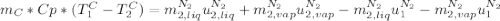
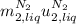 disappear because
disappear because 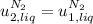 (The specific energy in the liquid is the same because the temperature does not change).
(The specific energy in the liquid is the same because the temperature does not change).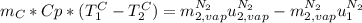
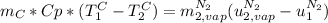
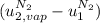 is the latent heat of vaporization because is the specific energy difference between the vapor and the liquid phases, so:
is the latent heat of vaporization because is the specific energy difference between the vapor and the liquid phases, so: