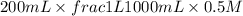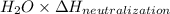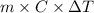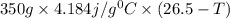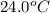Chemistry, 05.08.2019 19:10 camrenp9889
When 150. ml of 0.400 m h+ are mixed with 200. ml of 0.500 m oh-, the final temperature of the solution is 26.5°c. what was the initial temperature of the solution before the reaction occurred? assume that the solution has a total mass of 350. g and a specific heat capacity of 4.184 j/g°c. the enthalpy of neutralization for the reaction is -62.0 kj/mol of water produced.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3

Chemistry, 23.06.2019 10:00
Which number should be placed before f2 on the reactants side equation to make equation balanced? xe + > xef4
Answers: 1
You know the right answer?
When 150. ml of 0.400 m h+ are mixed with 200. ml of 0.500 m oh-, the final temperature of the solut...
Questions


Mathematics, 25.11.2019 06:31

Mathematics, 25.11.2019 06:31


Mathematics, 25.11.2019 06:31

Computers and Technology, 25.11.2019 06:31

History, 25.11.2019 06:31


English, 25.11.2019 06:31





Mathematics, 25.11.2019 06:31



Mathematics, 25.11.2019 06:31

Mathematics, 25.11.2019 06:31

English, 25.11.2019 06:31

Mathematics, 25.11.2019 06:31

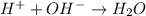
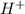 = volume × concentration of
= volume × concentration of 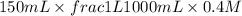
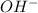 = volume × concentration of
= volume × concentration of