

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
What is the ph of an aqueous solution made by combining 35.81 ml of a 0.4364 m sodium acetate with 3...
Questions

Mathematics, 13.12.2020 02:10



Mathematics, 13.12.2020 02:10

Social Studies, 13.12.2020 02:10





Mathematics, 13.12.2020 02:10

Biology, 13.12.2020 02:10


Social Studies, 13.12.2020 02:10

Mathematics, 13.12.2020 02:10


History, 13.12.2020 02:10

Mathematics, 13.12.2020 02:10

Geography, 13.12.2020 02:10

Chemistry, 13.12.2020 02:10

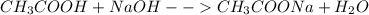
![pH=pKa+log\frac{[salt]}{[acid]}=4.74+log\frac{0.0158}{0.0119}=4.86](/tpl/images/0171/8130/95e51.png)


