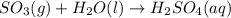Acid rain is produced from co2, and dissolving in atmospheric water molecules. this causes a decrease in the ph of water by increasing the h+ concentration. complete the reaction between sulfur trioxide and water in the formation of acid rain. include the phase of the acid produced.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3


Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
You know the right answer?
Acid rain is produced from co2, and dissolving in atmospheric water molecules. this causes a decre...
Questions

Chemistry, 20.10.2020 14:01

Mathematics, 20.10.2020 14:01


World Languages, 20.10.2020 14:01

Computers and Technology, 20.10.2020 14:01



English, 20.10.2020 14:01



Mathematics, 20.10.2020 14:01


History, 20.10.2020 14:01


Mathematics, 20.10.2020 14:01


Chemistry, 20.10.2020 14:01

Mathematics, 20.10.2020 14:01


Mathematics, 20.10.2020 14:01