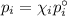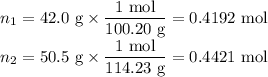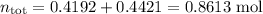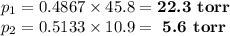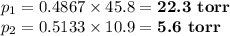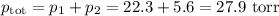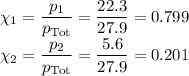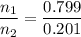Chemistry, 05.08.2019 21:20 DragonLovely
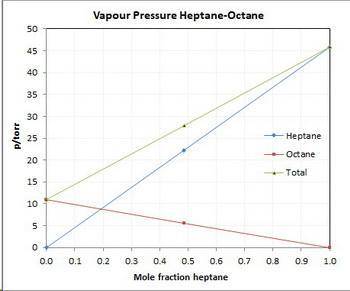
Asolution contains 42.0 g of heptane (c7h16) and 50.5 g of octane (c8h18) at 25 ∘c. the vapor pressures of pure heptane and pure octane at 25 ∘c are 45.8 torr and 10.9 torr, respectively. assuming ideal behavior, calculate each of the following. (note that the mole fraction of an individual gas component in an ideal gas mixture can be expressed in terms of the component's partial pressure.)a.) the vapor pressure of each of the solution components in the mixtureb.) the total pressure above the solutionc.) the composition of the vapor in mass percentd.) why is the composition of the vapor different from the composition of the solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1
You know the right answer?
Asolution contains 42.0 g of heptane (c7h16) and 50.5 g of octane (c8h18) at 25 ∘c. the vapor pressu...
Questions





English, 25.09.2019 19:20

English, 25.09.2019 19:20


English, 25.09.2019 19:20

English, 25.09.2019 19:20


English, 25.09.2019 19:20

English, 25.09.2019 19:20


Mathematics, 25.09.2019 19:20





English, 25.09.2019 19:20

English, 25.09.2019 19:20