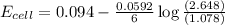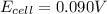Chemistry, 05.08.2019 22:20 baby092000
Consider the cell described below at 287 k: pb | pb2+ (1.07 m) || fe3+ (2.13 m) | fe given the standard reduction potentials found on the sheet attached to the exam, calculate the cell potential after the reaction has operated long enough for the [fe3+] to have changed by 1.052 m.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3
You know the right answer?
Consider the cell described below at 287 k: pb | pb2+ (1.07 m) || fe3+ (2.13 m) | fe given the stan...
Questions


Mathematics, 25.09.2019 14:40



Mathematics, 25.09.2019 14:40



Mathematics, 25.09.2019 14:40

Mathematics, 25.09.2019 14:40

Mathematics, 25.09.2019 14:40




History, 25.09.2019 14:40



Advanced Placement (AP), 25.09.2019 14:40



Mathematics, 25.09.2019 14:40

![E^0_{[Pb^{2+}/Pb]}=-0.13V](/tpl/images/0171/8681/82211.png)
![E^0_{[Fe^{3+}/Fe]}=-0.036V](/tpl/images/0171/8681/543db.png)
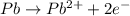
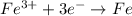
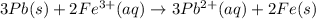
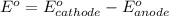
![E^o=E^o_{[Fe^{3+}/Fe]}-E^o_{[Pb^{2+}/Pb]}](/tpl/images/0171/8681/1c066.png)
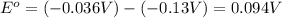
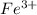 changed by 1.052 M.
changed by 1.052 M.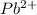 = 1.07 M
= 1.07 M![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Pb^{2+}]}{[Fe^{3+}]}](/tpl/images/0171/8681/72e91.png)
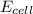 = emf of the cell = ?
= emf of the cell = ?