
Chemistry, 05.08.2019 22:20 gaberamos973
Consider the chemical reaction below: co + cl2 cocl2 k = 4.6 x 109how do the equilibrium concentration of the reactants compare to the equilibrium concentration of the product? they are much smaller. they are much bigger. they are about the same. they have to be exactly equal. you cannot tell from the information given.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1


Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
Consider the chemical reaction below: co + cl2 cocl2 k = 4.6 x 109how do the equilibrium concentrati...
Questions



Computers and Technology, 10.07.2019 15:30

Mathematics, 10.07.2019 15:30


Chemistry, 10.07.2019 15:30


Geography, 10.07.2019 15:30

English, 10.07.2019 15:30



Mathematics, 10.07.2019 15:30


Biology, 10.07.2019 15:40

Mathematics, 10.07.2019 15:40

Mathematics, 10.07.2019 15:40


Chemistry, 10.07.2019 15:40

Biology, 10.07.2019 15:40

Mathematics, 10.07.2019 15:40

 .
.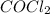 will be more.
will be more.

