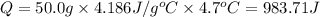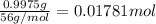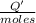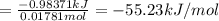Chemistry, 06.08.2019 01:20 larissacrystalow8g2w
0.9775 grams of an unknown compound is dissolved in 50.0 ml of water. initially the water temperature is 22.3 degrees celsius. after addition of the solid, the solution temperature is raised to about 27.0 degrees celsius. the substance is known to have a molar mass of about 56 g/mol. calculate the enthlapy of solution in kj/mol.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which statement justifies that phosphine (ph3) is a polar molecule?
Answers: 1

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1
You know the right answer?
0.9775 grams of an unknown compound is dissolved in 50.0 ml of water. initially the water temperatur...
Questions

Advanced Placement (AP), 08.04.2021 19:20



Biology, 08.04.2021 19:20

Mathematics, 08.04.2021 19:20


Biology, 08.04.2021 19:20


Mathematics, 08.04.2021 19:20


Mathematics, 08.04.2021 19:20




Mathematics, 08.04.2021 19:20

English, 08.04.2021 19:20


History, 08.04.2021 19:20

Computers and Technology, 08.04.2021 19:20

History, 08.04.2021 19:20