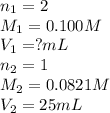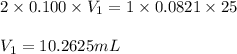Chemistry, 06.08.2019 01:30 heavendavis101
Sulfuric acid (h2so4) reacts with potassium hydroxide (koh) as follows. h2so4(aq) + 2 koh(aq) k2so4(aq) + 2 h2o(l) calculate the volume of 0.100 m sulfuric acid required to neutralize 25.0 ml of 0.0821 m koh.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
You know the right answer?
Sulfuric acid (h2so4) reacts with potassium hydroxide (koh) as follows. h2so4(aq) + 2 koh(aq) k2so4(...
Questions

Mathematics, 03.02.2021 22:00





Mathematics, 03.02.2021 22:00



Mathematics, 03.02.2021 22:00

Mathematics, 03.02.2021 22:00


Chemistry, 03.02.2021 22:00



Geography, 03.02.2021 22:00


Mathematics, 03.02.2021 22:00



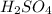 comes out to be 10.2625 mL.
comes out to be 10.2625 mL.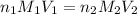
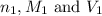 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 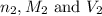 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.