
Chemistry, 06.08.2019 02:20 lindsay1054
For the reactionso2(g) + no2(g) so3(g) + no(g), the equilibrium constant is 18.0 at 1,200ºc. if 2.0 moles of so2 and 2.0 moles of no2 are placed in a 20. l container, what concentration of so3 will be present at equilibrium?
a) 0.081 mol/l
b) 0.019 mol/l
c) 0.11 mol/l
d) 1.00 mol/l
e) 18 mol/l

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3
You know the right answer?
For the reactionso2(g) + no2(g) so3(g) + no(g), the equilibrium constant is 18.0 at 1,200ºc. if 2.0...
Questions


Mathematics, 09.12.2021 04:40


History, 09.12.2021 04:40




Mathematics, 09.12.2021 04:40


Mathematics, 09.12.2021 04:40



Chemistry, 09.12.2021 04:40

Mathematics, 09.12.2021 04:40



Mathematics, 09.12.2021 04:40


Mathematics, 09.12.2021 04:40

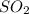 =
= 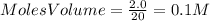
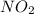 =
= 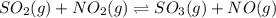
![K_c=\frac{[SO_3][NO]}{[SO_2][NO_2]}](/tpl/images/0172/0224/d13ef.png)
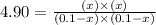
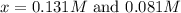
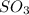 at equilibrium = x = 0.081 M
at equilibrium = x = 0.081 M

