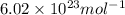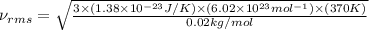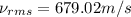Chemistry, 06.08.2019 02:20 mlittleduck6947
Consider neon, a noble gas whose molecules consist of single atoms of atomic mass 0.02 kg/mol. what is the average kinetic energy of a neon atom when the gas is at a temperature of 370 k? avogadro’s number is 6.02 × 1023 mol−1 and boltzmann’s constant is 1.38 × 10−23 j/k. answer in units of j. question 5, chap 19, sect 4. part 2 of 3 10 points what is the root mean square speed of a neon atom under such conditions? answer in units of m/s.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3


Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
Consider neon, a noble gas whose molecules consist of single atoms of atomic mass 0.02 kg/mol. what...
Questions


Mathematics, 03.05.2021 03:40

Mathematics, 03.05.2021 03:40

Mathematics, 03.05.2021 03:40

Mathematics, 03.05.2021 03:40


Health, 03.05.2021 03:40

Mathematics, 03.05.2021 03:40

Mathematics, 03.05.2021 03:40



Mathematics, 03.05.2021 03:40

Physics, 03.05.2021 03:40


Mathematics, 03.05.2021 03:50

History, 03.05.2021 03:50


Mathematics, 03.05.2021 03:50

Computers and Technology, 03.05.2021 03:50

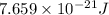
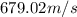
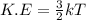


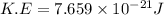
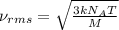
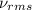 = root mean square speed
= root mean square speed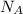 = Avogadro’s number =
= Avogadro’s number =