
Chemistry, 06.08.2019 04:20 kenzielema12
To a 25.00 ml volumetric flask, a lab technician adds a 0.150 g sample of a weak monoprotic acid, ha , and dilutes to the mark with distilled water. the technician then titrates this weak acid solution with 0.0969 m koh . she reaches the endpoint after adding 43.81 ml of the koh solution. determine the number of moles of the weak acid in the solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3

You know the right answer?
To a 25.00 ml volumetric flask, a lab technician adds a 0.150 g sample of a weak monoprotic acid, ha...
Questions

Biology, 20.10.2020 05:01



Business, 20.10.2020 05:01

English, 20.10.2020 05:01


Mathematics, 20.10.2020 05:01

English, 20.10.2020 05:01


Chemistry, 20.10.2020 05:01

Health, 20.10.2020 05:01



Mathematics, 20.10.2020 05:01

Mathematics, 20.10.2020 05:01

History, 20.10.2020 05:01

Mathematics, 20.10.2020 05:01



Mathematics, 20.10.2020 05:01

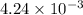 moles.
moles.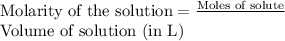
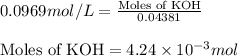
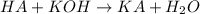
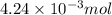 of KOH will react with =
of KOH will react with = 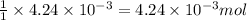 of weak monoprotic acid.
of weak monoprotic acid.

