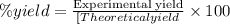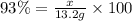Chemistry, 06.08.2019 05:10 cadanceowasso
When octane (c8h18) is burned in a particular internal combustion engine, the yield of carbon dioxide is 93%. what mass of carbon dioxide will be produced in this engine when 15.0 g of octane (mw = 114.0 g/mol) is burned with 15.0 g of oxygen gas (mw = 32.0 g/mol)? 2c8h18 + 25 o2 --> 16 co2 + 18 h2o
a.12g b.13g c.21g d.43g e.54g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3


Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
When octane (c8h18) is burned in a particular internal combustion engine, the yield of carbon dioxid...
Questions

Computers and Technology, 03.05.2021 15:20






Mathematics, 03.05.2021 15:20

Mathematics, 03.05.2021 15:20

Mathematics, 03.05.2021 15:20


Biology, 03.05.2021 15:20


Mathematics, 03.05.2021 15:20



Social Studies, 03.05.2021 15:20


Social Studies, 03.05.2021 15:20


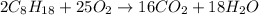
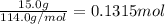
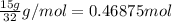
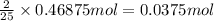 of octane
of octane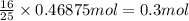 of carbon-dioxide
of carbon-dioxide