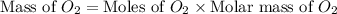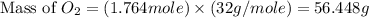Chemistry, 06.08.2019 05:30 lpslover26237
Glucose, c6h12o6, is used as an energy source by the human body. the overall reaction in the body is described by the equation c6h12o6(aq)+6o2(g)⟶6co2(g)+6h2o(l)
calculate the number of grams of oxygen required to convert 53.0 g of glucose to co2 and h2o.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
Glucose, c6h12o6, is used as an energy source by the human body. the overall reaction in the body is...
Questions








Computers and Technology, 20.12.2019 22:31












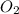 = 32 g/mole
= 32 g/mole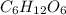 .
.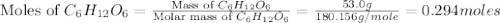
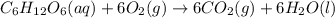
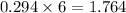 mole of
mole of