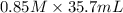Chemistry, 06.08.2019 06:10 vlactawhalm29
You have 185 ml of an acid with an unknown concentration. to determine the concentration you decide to use a 0.85 m solution of sodium hydroxide to titrate the acid. after the titration you determined that it took 35.7 ml of the 0.85 m solution of sodium hydroxid to reach the endpoint. what is the acids concentration?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
You have 185 ml of an acid with an unknown concentration. to determine the concentration you decide...
Questions

Mathematics, 12.02.2021 09:00

Mathematics, 12.02.2021 09:00

Health, 12.02.2021 09:00

Mathematics, 12.02.2021 09:00



Mathematics, 12.02.2021 09:00

Mathematics, 12.02.2021 09:00


Chemistry, 12.02.2021 09:00

Mathematics, 12.02.2021 09:00

Advanced Placement (AP), 12.02.2021 09:00


Chemistry, 12.02.2021 09:00


Mathematics, 12.02.2021 09:00

Mathematics, 12.02.2021 09:00

English, 12.02.2021 09:00


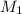 = ?,
= ?, 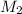 = 0.85 M
= 0.85 M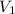 = 185 mL,
= 185 mL, 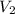 = 35.7 mL
= 35.7 mL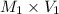 =
= 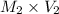
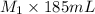 =
=