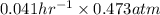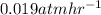Chemistry, 07.08.2019 00:10 KindaSmartPersonn
The breakdown of a certain pollutant x in sunlight is known to follow first-order kinetics. an atmospheric scientist studying the process fills a 20.0lreaction flask with a sample of urban air and finds that the partial pressure of x in the flask decreases from 0.473atm to 0.376atm over 5.6hours.
calculate the initial rate of decomposition of x, that is, the rate at which xwas disappearing at the start of the experiment.
round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
The breakdown of a certain pollutant x in sunlight is known to follow first-order kinetics. an atmos...
Questions


Social Studies, 29.10.2019 18:31




History, 29.10.2019 18:31





Mathematics, 29.10.2019 18:31




Mathematics, 29.10.2019 18:31


Mathematics, 29.10.2019 18:31


French, 29.10.2019 18:31

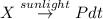
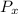
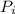 = initial partial pressure of X = 0.473 atm
= initial partial pressure of X = 0.473 atm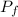 = final partial pressure of X = 0.376 atm
= final partial pressure of X = 0.376 atm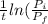
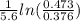
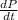 = k
= k