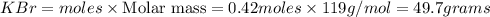Chemistry, 07.08.2019 17:10 risolatziyovudd
Calculate how many grams of the product form when 16.7 g of liquid bromine reacts with solid potassium. assume that there is more than enough of the solid potassium. 2 k(s) + br2(l) → 2 kbr(s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
Calculate how many grams of the product form when 16.7 g of liquid bromine reacts with solid potassi...
Questions

English, 04.11.2020 03:10

Social Studies, 04.11.2020 03:10

English, 04.11.2020 03:10

Social Studies, 04.11.2020 03:10



Biology, 04.11.2020 03:10


Law, 04.11.2020 03:10



English, 04.11.2020 03:10

Mathematics, 04.11.2020 03:10


Mathematics, 04.11.2020 03:10

Mathematics, 04.11.2020 03:10

Mathematics, 04.11.2020 03:10

Chemistry, 04.11.2020 03:10

Mathematics, 04.11.2020 03:10

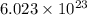 of particles.
of particles.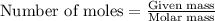
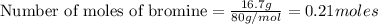
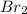 acts as limiting reagent as it limits the formation of product as potassium is in excess.
acts as limiting reagent as it limits the formation of product as potassium is in excess.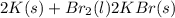
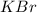
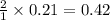 moles of
moles of