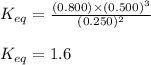Chemistry, 07.08.2019 17:20 kellymcdow9385
For the reaction 2nh3(g)↽−−⇀ 3h2(g)+n2(g) the equilibrium concentrations were found to be [nh3]=0.250 m , [h2]=0.500 m , and [n2]=0.800 m . what is the equilibrium constant for this reaction?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 10:30
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2
You know the right answer?
For the reaction 2nh3(g)↽−−⇀ 3h2(g)+n2(g) the equilibrium concentrations were found to be [nh3]=0.25...
Questions











Social Studies, 23.07.2021 04:40






Chemistry, 23.07.2021 04:40

Mathematics, 23.07.2021 04:40


Biology, 23.07.2021 04:40

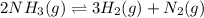
![K_{eq}=\frac{[N_2][H_2]^3}{[NH_3]^2}](/tpl/images/0172/7166/804f3.png)
![[NH_3]=0.250M](/tpl/images/0172/7166/0d43e.png)
![[H_2]=0.500M](/tpl/images/0172/7166/5e4b7.png)
![[N_2]=0.800M](/tpl/images/0172/7166/3aba5.png)