
Chemistry, 08.08.2019 00:20 aroland1990x
Calculate the δ h°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide. δ h°f [caco3(s)] = –1206.9 kj/mol; δ h°f [cao(s)] = –635.1 kj/mol; δ h°f [co2(g)] = –393.5 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2
You know the right answer?
Calculate the δ h°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide...
Questions

English, 10.02.2020 22:41

Social Studies, 10.02.2020 22:41

Social Studies, 10.02.2020 22:41

Mathematics, 10.02.2020 22:41






English, 10.02.2020 22:42









Social Studies, 10.02.2020 22:42


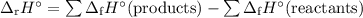
![\begin{array}{rcl}\Delta_{\text{r}}H^{\circ}& = & [(-635.1 + (-393.5)] - (-1206.9)\\& = & -1028.6 +1206.9\\& = & \mathbf{178.3}\\\end{array}\\\text{The enthalpy of decomposition is } \boxed{\textbf{178.3 kJ/mol}}](/tpl/images/0172/9753/c40b5.png) l}}
l}}

