Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
By titration, it is found that 95.5 ml95.5 ml of 0.188 m naoh(aq)0.188 m naoh(aq) is needed to neutr...
Questions



English, 07.04.2021 05:50




Geography, 07.04.2021 05:50







Chemistry, 07.04.2021 05:50






Mathematics, 07.04.2021 05:50


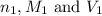 are the n-factor, molarity and volume of acid which is HCl
are the n-factor, molarity and volume of acid which is HCl are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.