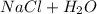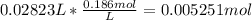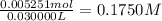Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
A30.00-ml sample of hydrochloric acid solution requires 28.23 ml of o.186 m sodium hydroxide for com...
Questions

Mathematics, 22.04.2020 17:26

Mathematics, 22.04.2020 17:26

Biology, 22.04.2020 17:26



Mathematics, 22.04.2020 17:26

Spanish, 22.04.2020 17:26





Mathematics, 22.04.2020 17:26

Biology, 22.04.2020 17:26

Mathematics, 22.04.2020 17:27

Mathematics, 22.04.2020 17:27





 ⇒
⇒