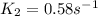Chemistry, 08.08.2019 05:30 sparky1234
Areaction is found to have an activation energy of 108 kj/mol. if the rate constant for this reaction is 4.60 x 10-6 s-1 at 275 k, what is the rate constant at 366 k? 0.58 1/s

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
Areaction is found to have an activation energy of 108 kj/mol. if the rate constant for this reactio...
Questions

Chemistry, 22.10.2020 02:01

Chemistry, 22.10.2020 02:01

Biology, 22.10.2020 02:01

Biology, 22.10.2020 02:01


Mathematics, 22.10.2020 02:01

Advanced Placement (AP), 22.10.2020 02:01

English, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01


English, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01



Social Studies, 22.10.2020 02:01

History, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01

World Languages, 22.10.2020 02:01

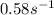
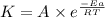
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0173/1540/6d953.png)
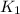 =rate constant at
=rate constant at 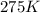 =
= 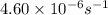
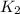 = rate constant at
= rate constant at 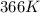 = ?
= ?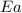 = activation energy for the reaction = 108kJ/mol=108000 J/mol
= activation energy for the reaction = 108kJ/mol=108000 J/mol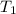 = initial temperature =
= initial temperature = 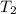 = final temperature =
= final temperature = ![\log (\frac{K_2}{4.60\times 10^{-6}})=\frac{108000}{2.303\times 8.314J/mole.K}[\frac{1}{275K}-\frac{1}{366K}]](/tpl/images/0173/1540/60a14.png)