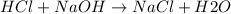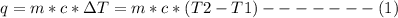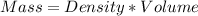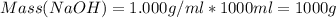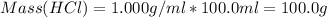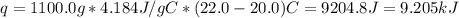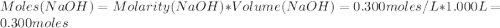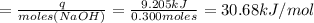Chemistry, 08.08.2019 06:10 joshuahinton45
A1000 ml sample of 0.300 m naoh is mixed with 100.0 ml of 0.300 m hcl in a coffee cup calorimeter. if both solutions are at 20.0 °c and the final temperature of the mixture was 22.0 °c find the heat of neutralization, ahreu in kj/mole. assume no heat is lost to the surroundings, the density of all solutions is 1.00 g/ml, and c, of the mixture is 4.184 j/g·°c

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

You know the right answer?
A1000 ml sample of 0.300 m naoh is mixed with 100.0 ml of 0.300 m hcl in a coffee cup calorimeter. i...
Questions

History, 07.04.2021 20:10

English, 07.04.2021 20:10


Mathematics, 07.04.2021 20:10



Mathematics, 07.04.2021 20:10


History, 07.04.2021 20:10

Mathematics, 07.04.2021 20:10







Mathematics, 07.04.2021 20:10


Biology, 07.04.2021 20:10

Chemistry, 07.04.2021 20:10