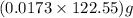8. a sample of potassium chlorate (kcio,) was heated in a test tube and decomposed 2kc? (s) 302 (g) + 2kcio, (s) the oxygen was collected by the displacement of water at 22'c at a total pressure of 754 torr. the volume of the gas collected was 0.65l and the vapor pressure of water at 22°c is 21 torr. calculate a) the partial pressure of o2 in the gas collected and b) the mass of kcio3 in the sample that was decomposed

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2


You know the right answer?
8. a sample of potassium chlorate (kcio,) was heated in a test tube and decomposed 2kc? (s) 302 (g)...
Questions




Biology, 16.02.2022 07:50

English, 16.02.2022 07:50

Biology, 16.02.2022 07:50




Business, 16.02.2022 07:50




SAT, 16.02.2022 07:50

Mathematics, 16.02.2022 07:50

English, 16.02.2022 07:50


Mathematics, 16.02.2022 07:50


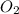 in the gas was 733 torr and mass of
in the gas was 733 torr and mass of 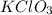 in the sample was 2.12 g.
in the sample was 2.12 g.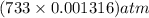 = 0.9646 atm
= 0.9646 atm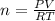
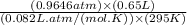
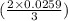 moles or 0.0173 moles of
moles or 0.0173 moles of