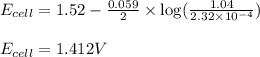Chemistry, 08.08.2019 06:10 david1236544
what is the calculated value of the cell potential at 298k for an electrochemical cell with the following reaction, when the cu2+ concentration is 2.32×10-4 m and the mn2+ concentration is 1.04 m ?
cu2+(aq) + mn(s) cu(s) + mn2+(aq)
the cell reaction as written above is spontaneous for the concentrations given: (true/false)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Covalent bonds are formed between metals and boiling points true or false
Answers: 2

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1
You know the right answer?
what is the calculated value of the cell potential at 298k for an electrochemical cell with the foll...
Questions


Advanced Placement (AP), 27.01.2021 17:30


Arts, 27.01.2021 17:30





History, 27.01.2021 17:30


Mathematics, 27.01.2021 17:30


Social Studies, 27.01.2021 17:30



Engineering, 27.01.2021 17:30

Mathematics, 27.01.2021 17:30


Biology, 27.01.2021 17:30

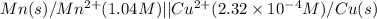
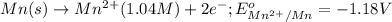
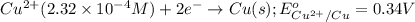
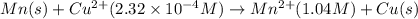
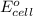 of the reaction, we use the equation:
of the reaction, we use the equation: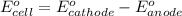
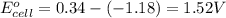
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Mn^{2+}]}{[Cu^{2+}]}](/tpl/images/0173/1697/70482.png)
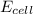 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V![[Cu^{2+}]=2.32\times 10^{-4}M](/tpl/images/0173/1697/f12ce.png)
![[Mn^{2+}]=1.04M](/tpl/images/0173/1697/250ae.png)