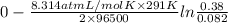Aconcentration cell is built based on the following half reactions by using two pieces of zinc as electrodes, two zn2+ solutions, 0.129 m and 0.427 m, and all other materials needed for a galvanic cell. what will the potential of this cell be when the cathode concentration of zn2+ has changed by 0.047 m at 291 k?
zn2+ + 2 e- ? zn eo = -0.761 v

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
Aconcentration cell is built based on the following half reactions by using two pieces of zinc as el...
Questions

Biology, 17.03.2020 16:29


Law, 17.03.2020 16:29



Computers and Technology, 17.03.2020 16:29


Mathematics, 17.03.2020 16:29





Geography, 17.03.2020 16:30


Mathematics, 17.03.2020 16:30



Mathematics, 17.03.2020 16:30


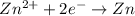 ,
, 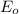 = -0.761 V
= -0.761 V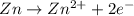 ,
, 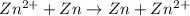
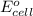 for the given reaction is zero.
for the given reaction is zero.![E^{o}_{cell} - \frac{RT}{nF} ln \frac{[Zn^{2+}]_{products}}{[Zn^{2+}]_{reactants}}](/tpl/images/0173/1699/4d9c9.png)