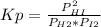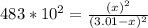Chemistry, 08.08.2019 06:20 sullivanjakob
At a particular temperature, kp-483 × 102 for the reaction h2 (g) + 12(g) ? 2h1(g) if 3 01 atm of h-(g) and 3.01 atm of i2(g) are introduced into a 1.00-l- container, calculate the equilibrium partial pressures of all partial pressure of h2 partial pressure of i2atm partial pressure ofhiatm atm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3
You know the right answer?
At a particular temperature, kp-483 × 102 for the reaction h2 (g) + 12(g) ? 2h1(g) if 3 01 atm of h...
Questions

Mathematics, 09.10.2019 15:30


Health, 09.10.2019 15:30

History, 09.10.2019 15:30





History, 09.10.2019 15:30

Social Studies, 09.10.2019 15:30

Physics, 09.10.2019 15:30

Mathematics, 09.10.2019 15:30

Social Studies, 09.10.2019 15:30

Social Studies, 09.10.2019 15:30





Mathematics, 09.10.2019 15:30

Physics, 09.10.2019 15:30