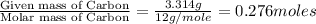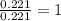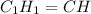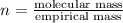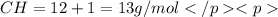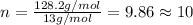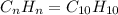Chemistry, 08.08.2019 06:20 alwaysneedhelp84
when 3.539 grams of a hydrocarbon, cxhy, were burned in a combustion analysis apparatus, 12.15 grams of co2 and 1.990 grams of h2o were produced.
in a separate experiment, the molar mass of the compound was found to be 128.2 g/mol. determine the empirical formula and the molecular formula of the hydrocarbon.
enter the elements in the order presented in the question.
empirical formula =
molecular formula =

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1


Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2
You know the right answer?
when 3.539 grams of a hydrocarbon, cxhy, were burned in a combustion analysis apparatus, 12.15 grams...
Questions

Mathematics, 18.03.2021 06:20






Mathematics, 18.03.2021 06:20


History, 18.03.2021 06:20

Mathematics, 18.03.2021 06:20

Mathematics, 18.03.2021 06:20

Chemistry, 18.03.2021 06:20

English, 18.03.2021 06:20

History, 18.03.2021 06:20

Mathematics, 18.03.2021 06:20

Health, 18.03.2021 06:20

Social Studies, 18.03.2021 06:20


Mathematics, 18.03.2021 06:20

Mathematics, 18.03.2021 06:20

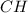 and
and 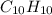 respectively.
respectively.
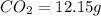
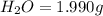
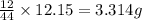 of carbon will be contained.
of carbon will be contained.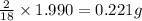 of hydrogen will be contained.
of hydrogen will be contained.