

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Write a net ionic equation for the overall reaction that occurs when aqueous solutions of sodium hyd...
Questions


Mathematics, 05.05.2020 23:58

Biology, 05.05.2020 23:58


Biology, 05.05.2020 23:58




Mathematics, 05.05.2020 23:58



Mathematics, 05.05.2020 23:58




Health, 05.05.2020 23:58



Mathematics, 05.05.2020 23:58



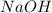 while the molecular formula for oxalic acid is given as (
while the molecular formula for oxalic acid is given as (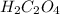 )
)

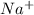 is the sodium ion
is the sodium ion 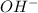 is the hydroxide ion
is the hydroxide ion 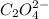 is the oxalate ion
is the oxalate ion

