
Chemistry, 08.08.2019 19:10 alyxkellar06
Under certain conditions, the equilibrium constant of the reaction below is kc=1.7×10−3. if the reaction begins with a concentration of 0.0546 m for each of sbcl3 and cl2 and a concentration of 0.0 m for sbcl5, what is the equilibrium concentration (in molarity) of cl2? sbcl5(g)↽−−⇀sbcl3(g)+cl2(g)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2
You know the right answer?
Under certain conditions, the equilibrium constant of the reaction below is kc=1.7×10−3. if the reac...
Questions


English, 02.11.2020 23:50

Mathematics, 02.11.2020 23:50

Mathematics, 02.11.2020 23:50

History, 02.11.2020 23:50

Mathematics, 02.11.2020 23:50








English, 02.11.2020 23:50

English, 02.11.2020 23:50


Mathematics, 02.11.2020 23:50

Mathematics, 02.11.2020 23:50


Mathematics, 02.11.2020 23:50

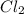 at equilibrium will be, 0.0086 M
at equilibrium will be, 0.0086 M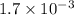
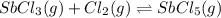
![K_c=\frac{[SbCl_5]}{[SbCl_3][Cl_2]}](/tpl/images/0173/2882/257e5.png)
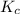 will be,
will be, 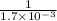
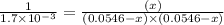
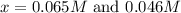
![(0.0546-x)M=[0.0546-2(0.045)]M=0.0086M](/tpl/images/0173/2882/293d4.png)


