
The decomposition of nh4hs is endothermic: nh4hs(s)⇌nh3(g)+h2s(g) part a which change to an equilibrium mixture of this reaction results in the formation of more h2s? which change to an equilibrium mixture of this reaction results in the formation of more ? a decrease in the volume of the reaction vessel (at constant temperature) an increase in the amount of nh4hs in the reaction vessel an increase in temperature all of the above

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1


You know the right answer?
The decomposition of nh4hs is endothermic: nh4hs(s)⇌nh3(g)+h2s(g) part a which change to an equilib...
Questions


Physics, 26.03.2020 19:10

English, 26.03.2020 19:10





Computers and Technology, 26.03.2020 19:10

Mathematics, 26.03.2020 19:10






History, 26.03.2020 19:10


English, 26.03.2020 19:10

Mathematics, 26.03.2020 19:10


Mathematics, 26.03.2020 19:10

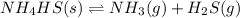
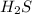 will increase.
will increase.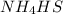 then equilibrium will shift in the direction of decrease in concentration that is, in the forward direction.
then equilibrium will shift in the direction of decrease in concentration that is, in the forward direction.

