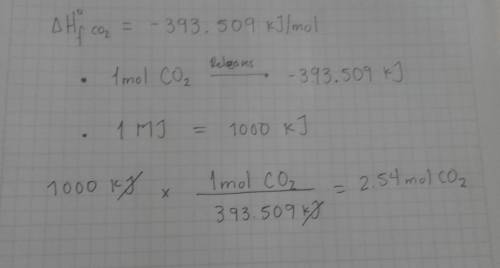Many power plants produce energy by burning carbon-based fuels, which also produces carbon dioxide. carbon dioxide is a greenhouse gas, so over-production can have negative effects on the environment. use enthalpy of formation data to calculate the number of moles of co2(g) produced per megajoule of heat released from the combustion of each fuel under standard conditions (1 atm and 25 °c).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
Many power plants produce energy by burning carbon-based fuels, which also produces carbon dioxide....
Questions


History, 04.10.2020 14:01


English, 04.10.2020 14:01



Mathematics, 04.10.2020 14:01


History, 04.10.2020 14:01


Physics, 04.10.2020 14:01



History, 04.10.2020 14:01

Mathematics, 04.10.2020 14:01

English, 04.10.2020 14:01



History, 04.10.2020 14:01