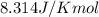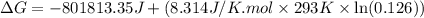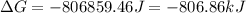Chemistry, 09.08.2019 16:20 estheradame547
Methane, a component in natural gas, can be used as a fuel in combustion reactions. what is the value for δgnon (in kj) for the following reaction under the given conditions at 293 k? ch4 (g) + 2 o2 (g) → co2 (g) + 2 h2o (g) where δhorxn = -803 kj, δsorxn = -4.05 j/k, and [ch4] = 14.51 m, [o2] = 9.27 m, [co2] = 3.83 m, and [h2o] = 6.41 m. (in kj)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Methane, a component in natural gas, can be used as a fuel in combustion reactions. what is the valu...
Questions


Mathematics, 27.02.2021 03:30

Chemistry, 27.02.2021 03:30



Social Studies, 27.02.2021 03:30


Mathematics, 27.02.2021 03:30

Biology, 27.02.2021 03:30

Mathematics, 27.02.2021 03:30




Geography, 27.02.2021 03:30


Mathematics, 27.02.2021 03:30

Mathematics, 27.02.2021 03:30

Social Studies, 27.02.2021 03:30


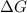 for the reaction is -806.86 kJ
for the reaction is -806.86 kJ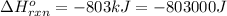 (Conversion factor: 1 kJ = 1000)
(Conversion factor: 1 kJ = 1000)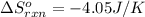
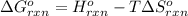
![\Delta G^o_{rxn}=-803000J-[(293K)\times (-4.05J/K)]=-801813.35J](/tpl/images/0173/7106/202ef.png)
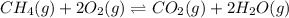
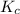 is given as:
is given as:![K_{c}=\frac{[H_2O]^2[CO_2]}{[CH_4][O_2]^2}](/tpl/images/0173/7106/38d41.png)
![[H_2O]=6.41M](/tpl/images/0173/7106/c8ec5.png)
![[CO_2]=3.83M](/tpl/images/0173/7106/f564f.png)
![[CH_4]=14.51M](/tpl/images/0173/7106/7f12b.png)
![[O_2]=9.27M](/tpl/images/0173/7106/50f67.png)
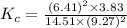
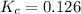
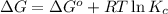
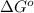 = Standard gibbs' free energy change of the reaction = -801813.35 J
= Standard gibbs' free energy change of the reaction = -801813.35 J