Chemistry, 09.08.2019 17:20 jgnjanjdknsjrr9358
Given the concentrations, calculate the equilibrium constant for this reaction: pcl3(g) +cl2(g)⇌pcl5(g) at equilibrium, the molar concentrations for reactants and products are found to be [pcl3]=0.20 m, [cl2]=0.25 m, and [pcl5]=1.20 m. what is the equilibrium constant (kc) for this reaction? express your answer using two significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1


Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2
You know the right answer?
Given the concentrations, calculate the equilibrium constant for this reaction: pcl3(g) +cl2(g)⇌pcl...
Questions



English, 06.05.2021 17:40

Biology, 06.05.2021 17:40




Computers and Technology, 06.05.2021 17:40



Mathematics, 06.05.2021 17:40

Mathematics, 06.05.2021 17:40

Mathematics, 06.05.2021 17:40


Mathematics, 06.05.2021 17:40

Mathematics, 06.05.2021 17:40

English, 06.05.2021 17:40

Geography, 06.05.2021 17:40



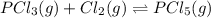
![K_c=\frac{[PCl_5]}{[PCl_3][Cl_2]}](/tpl/images/0173/7310/4c8d0.png)