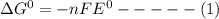Consider the following half reaction in which nitrate is reduced to nitrite: no3– 2h 2e– → no2– h2o. the reaction has a δg°' value of –81 kj/mol. the negative value of δg°' results from the very e°' value of the redox couple no3–/no2–, and it reflects the strong tendency of nitrate to electrons. choose one: a. positive / donateb. negative/ acceptc. negative / donated. positive / accept

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Consider the following half reaction in which nitrate is reduced to nitrite: no3– 2h 2e– → no2– h2o...
Questions

Computers and Technology, 27.08.2019 15:30

Mathematics, 27.08.2019 15:30

World Languages, 27.08.2019 15:30




Mathematics, 27.08.2019 15:30






English, 27.08.2019 15:30

Physics, 27.08.2019 15:30



History, 27.08.2019 15:30

Geography, 27.08.2019 15:30

History, 27.08.2019 15:30

Arts, 27.08.2019 15:30