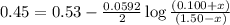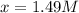Chemistry, 09.08.2019 21:20 thatcurlysophia
77. a voltaic cell consists of a zn/zn2+ half-cell and a ni/ni2+ half-cell at 25°c. the initial concentrations of ni2+ and zn2+ are 1.50 m and 0.100 m, respectively. a. what is the initial cell potential? b. what is the cell potential when the concentration of ni2+ has fallen to 0.500 m? c. what are the concentrations of ni2+ and zn2+ when the cell potential falls to 0.45 v?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2
You know the right answer?
77. a voltaic cell consists of a zn/zn2+ half-cell and a ni/ni2+ half-cell at 25°c. the initial conc...
Questions


Physics, 08.12.2021 16:30

Mathematics, 08.12.2021 16:30



Arts, 08.12.2021 16:30



English, 08.12.2021 16:30

Social Studies, 08.12.2021 16:30




History, 08.12.2021 16:30


Biology, 08.12.2021 16:30


English, 08.12.2021 16:40

Chemistry, 08.12.2021 16:40

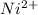 has fallen to 0.500 M is, 0.52 V
has fallen to 0.500 M is, 0.52 V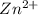 when the cell potential falls to 0.45 V are, 0.01 M and 1.59 M
when the cell potential falls to 0.45 V are, 0.01 M and 1.59 M![E^0_{[Ni^{2+}/Ni]}=-0.23V](/tpl/images/0173/8654/be6be.png)
![E^0_{[Zn^{2+}/Zn]}=-0.76V](/tpl/images/0173/8654/4cd18.png)
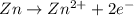
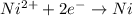
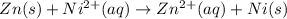
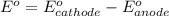
![E^o=E^o_{[Ni^{2+}/Ni]}-E^o_{[Zn^{2+}/Zn]}](/tpl/images/0173/8654/7d468.png)
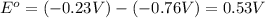
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}]}{[Ni^{2+}]}](/tpl/images/0173/8654/c02a9.png)
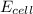 = emf of the cell = ?
= emf of the cell = ?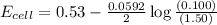
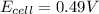
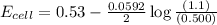
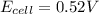
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}+x]}{[Ni^{2+}-x]}](/tpl/images/0173/8654/e92ff.png)