

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3

You know the right answer?
For the reaction 2 a ⇌ b, the equilibrium concentrations are as follows: [a] = 0.056 mand [b] = 0.1...
Questions

Mathematics, 05.05.2020 22:33


Biology, 05.05.2020 22:33

Social Studies, 05.05.2020 22:33

Mathematics, 05.05.2020 22:33



History, 05.05.2020 22:34


Spanish, 05.05.2020 22:34





Mathematics, 05.05.2020 22:34



Mathematics, 05.05.2020 22:34

Social Studies, 05.05.2020 22:34

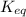 .
.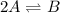
![K_{eq} = \frac{[B]}{[A]^{2}}](/tpl/images/0173/8965/36e57.png)
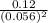
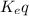 ) for the reaction is 38.
) for the reaction is 38.

