
Chemistry, 10.08.2019 00:10 makennskyee1198
If the reaction n2 (g) + 3 h2 (g) --> 2 nh3 (g) has the concentrations 1.1 m for nitrogen, 0.75 m for hydrogen and 0.25 m for ammonia gas, what is the kc? show all work. does this mean that there are more reactants or products at equilibrium? explain how you determined that.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
If the reaction n2 (g) + 3 h2 (g) --> 2 nh3 (g) has the concentrations 1.1 m for nitrogen, 0.75...
Questions


Computers and Technology, 17.01.2020 19:31

Business, 17.01.2020 19:31






Mathematics, 17.01.2020 19:31








Computers and Technology, 17.01.2020 19:31



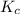 is 0.136 and is reactant favored.
is 0.136 and is reactant favored.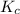
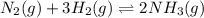
![K_{c}=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0173/9631/f3b94.png)
![[NH_3]=0.25M](/tpl/images/0173/9631/306b2.png)
![[H_2]=0.75M](/tpl/images/0173/9631/5c336.png)
![[N_2]=1.1M](/tpl/images/0173/9631/4c5ce.png)
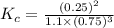
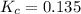
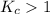 ; the reaction is product favored.When
; the reaction is product favored.When 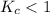 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 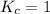 ; the reaction is in equilibrium.
; the reaction is in equilibrium.

