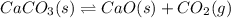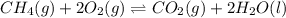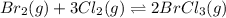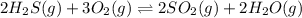Chemistry, 10.08.2019 00:10 dheydar3506
Each of the following reactions is allowed to reach equilibrium in a sealed container. for which of the reactions could you shift the equilibrium to the right by decreasing the pressure?
ch4(g) + 2o2(g) double arrow yields co2(g) + 2h2o(l)
caco3(s) double arrow yields cao(s) + co2(g)
br2(g) + 3cl2(g) double arrow yields 2brcl3(g)
2h2s(g) + 3o2(g) double arrow yields 2so2(g) + 2h2o(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1
You know the right answer?
Each of the following reactions is allowed to reach equilibrium in a sealed container. for which of...
Questions

Biology, 30.04.2021 22:40




Mathematics, 30.04.2021 22:40

Mathematics, 30.04.2021 22:40



Mathematics, 30.04.2021 22:40

Chemistry, 30.04.2021 22:40


Mathematics, 30.04.2021 22:40

Physics, 30.04.2021 22:40







Geography, 30.04.2021 22:40