
Suppose an epa chemist tests a 200.ml sample of groundwater known to be contaminated with iron(ii) chloride, which would react with silver nitrate solution like this: fecl2 (aq) + 2agno3 (aq) → 2agcl (s) + feno32 (aq) the chemist adds 14.0m m silver nitrate solution to the sample until silver chloride stops forming. she then washes, dries, and weighs the precipitate. she finds she has collected 6.9mg of silver chloride. calculate the concentration of iron(ii) chloride contaminant in the original groundwater sample. be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1
You know the right answer?
Suppose an epa chemist tests a 200.ml sample of groundwater known to be contaminated with iron(ii) c...
Questions


Mathematics, 12.01.2021 19:50

Mathematics, 12.01.2021 19:50

Arts, 12.01.2021 19:50


Business, 12.01.2021 19:50

Business, 12.01.2021 19:50


Mathematics, 12.01.2021 19:50

Health, 12.01.2021 19:50

Arts, 12.01.2021 19:50

Mathematics, 12.01.2021 19:50


Mathematics, 12.01.2021 19:50


Mathematics, 12.01.2021 19:50



Mathematics, 12.01.2021 19:50

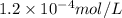 .
.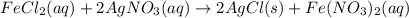
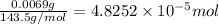
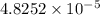 moles of silver chloride will be obtained from:
moles of silver chloride will be obtained from: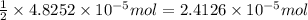
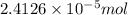
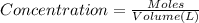
![[FeCl_2]=\frac{2.4126\times 10^{-5} mol}{0.2 L}=0.00012063 mol/L](/tpl/images/0173/9764/69c0f.png)
![[FeCl_2]=1.2063\times 10^{-4} mol/L\approx 1.2\times 10^{-4} mol/L](/tpl/images/0173/9764/77e47.png)


