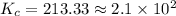Chemistry, 10.08.2019 01:20 mikemurray115
The molar concentrations for the reactants and products at equilibrium are found to be [ccl4]=1.0 m, [o2]=0.3 m, [cocl2]=4.0 m, and [cl2]=2.0 m. what is the value of the equilibrium constant for this reaction? 2ccl4(g)+o2(g)⇌2cocl2(g)+2cl2(g) express your answer numerically using two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
The molar concentrations for the reactants and products at equilibrium are found to be [ccl4]=1.0 m,...
Questions

Mathematics, 28.01.2021 17:20

Health, 28.01.2021 17:20


History, 28.01.2021 17:20

Mathematics, 28.01.2021 17:20



Mathematics, 28.01.2021 17:20

Mathematics, 28.01.2021 17:20

Mathematics, 28.01.2021 17:20

English, 28.01.2021 17:20

Social Studies, 28.01.2021 17:20


Mathematics, 28.01.2021 17:20


Mathematics, 28.01.2021 17:20



Social Studies, 28.01.2021 17:20

English, 28.01.2021 17:20

 is the value of the equilibrium constant for this reaction.
is the value of the equilibrium constant for this reaction.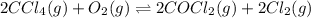
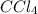 at equilibrium =
at equilibrium =![[CCl_4]=1.0M](/tpl/images/0174/0028/d343b.png)
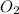 at equilibrium =
at equilibrium =![[O_2]=0.3M](/tpl/images/0174/0028/056b2.png)
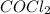 at equilibrium =
at equilibrium =![[COCl_2]=4.0M](/tpl/images/0174/0028/945ea.png)
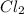 at equilibrium =
at equilibrium =![[Cl_2]=2.0M](/tpl/images/0174/0028/565ad.png)
![K_c=\frac{[COCl_2]^2[Cl_2]^2}{[CCl_4]^2[O_2]}=\frac{(4.0 M)^2\times (2.0M)^2}{(1.0M)^2\times (0.3 M)}](/tpl/images/0174/0028/1316e.png)